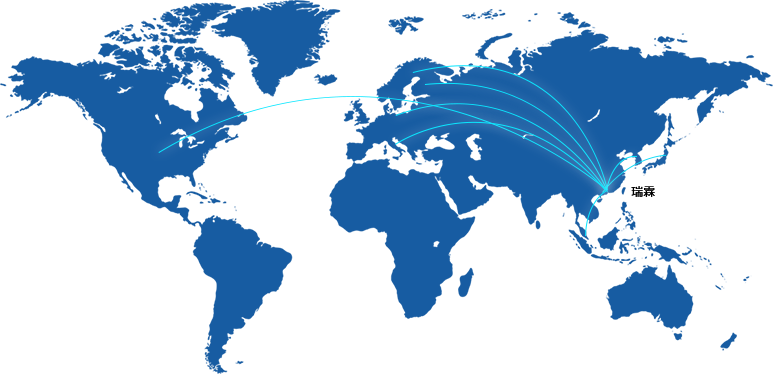
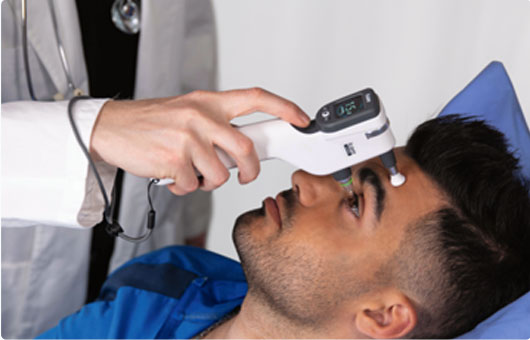
Relin Medicine focuses on specialty products that are both distinctive and competitive in the market. Currently, the company's main business area is ophthalmology, and it is actively expanding into fields such as otolaryngology. It’s partners are located in various countries including China, the United States, Germany, Switzerland, Japan, South Korea, Singapore, Finland, and Italy, etc.
Relin Medicine can provide one-stop services to partners, including market research, product registration, import and commercial distribution, governmental affairs, sales promotion, etc. Relin Medicine’s cooperation modes are diverse and open, ranging from a single general agent model to multiple modes such as project introduction, technology transfer, collaborative development, and contract manufacturing.
Relin Medicine sincerely looks forward to working with you. If you are interested in discussing potential projects, please contact our Business Development Department:
Tel: 86-755-25232061
Note: Other partners such as channels, operators, and technology partners are not reflected in this section.




 Home >
Home >